CMMS for GMP Compliance: What Auditors Look for in Digital Records
Good Manufacturing Practice (GMP) auditors scrutinize maintenance documentation with unrelenting precision, searching for gaps that reveal whether equipment supporting regulated production remains under actual control. Over 50% of all FDA citations relate to inadequate procedures, with data integrity and incomplete records remaining as perennial problems that transform routine audits into enforcement actions. For pharmaceutical, food, and beverage manufacturers operating under GMP regulations, maintenance records represent primary evidence that production systems remain qualified, controlled, and fit for purpose.
This article examines how CMMS for GMP compliance transforms scattered maintenance practices into verified regulatory proof through GMP maintenance systems, GMP digital records that satisfy ALCOA+ principles, and the GMP maintenance documentation auditors expect during inspections.
Why GMP Auditors Focus Heavily on Maintenance Documentation
Auditors assess whether maintenance records demonstrate control, traceability, and readiness for regulated production, because poorly maintained equipment represents a direct compliance risk that threatens product quality. Disconnected spreadsheets, version conflicts, and missing timestamps create gaps that raise concerns about data integrity compliance, accountability, and equipment reliability, which compound during inspections.

With comprehensive maintenance management platforms, teams present unified, inspection-ready documentation that auditors can review without ambiguity through validated maintenance procedures. This digital approach ensures that equipment traceability connects every maintenance event to the asset qualification status, eliminating the documentation gaps that turn observations into actionable findings.
The Key Maintenance Records GMP Auditors Expect to Verify
Complete Equipment Histories
Auditors review whether maintenance actions, inspections, and corrective interventions demonstrate continuous control over regulated equipment throughout its lifecycle. Histories must clearly show what happened, when it happened, why it happened, and who performed or reviewed the work to satisfy regulatory scrutiny. Compliance failures have resulted in $1.1 billion in penalties over the past five years, with incomplete equipment documentation contributing significantly to these enforcement actions.
| Equipment History element | Auditor Expectation | CMMS Capability |
|---|---|---|
| Maintenance Event Details | Complete scope, parts used, results | Standardized digital work orders |
| Technician Attribution | Verified identity and qualifications | Role-based access with certifications |
| Timing Documentation | Contemporaneous recording | Automated timestamps at capture |
| Review Approval | Supervisor verification required | Mandatory digital approval workflows |
Records must be attributable, legible, contemporaneous, original, and accurate to meet ALCOA+ data integrity principles that regulators increasingly emphasize during inspections. A modern platform captures these elements automatically through asset history logs, ensuring documentation supports both operational needs and compliance verification without manual transcription which can introduce errors.
Maintenance Intervals Supported by Validated Schedules
GMP requires maintenance to occur at defined frequencies established during validation and risk assessments, which prove that the equipment remains at a qualified status. Inspectors look for overdue tasks, skipped tasks, and justification of any deviations from defined schedules that could compromise equipment qualification. Organizations experience an average of 8.6% value erosion during operational lifecycles due to poor schedule management, including maintenance timing that affects equipment reliability.
| Schedule Control Element | Without Automation | With CMMS Automation |
|---|---|---|
| PM Frequency Definition | Spreadsheets, easily modified | Locked schedules requiring change control |
| Overdue Task Visibility | Discovered during audits | Flagged immediately through alerts |
| Deviation Justification | After-the-fact documentation | Formal approval before schedule changes |
| Maintenance Scheduling Accuracy | Manual tracking, errors are common | System-enforced intervals with alerts |
Schedules must be documented, accessible, and protected from unauthorized modification through maintenance documentation controls. Using a CMMS for GMP compliance, however, allows teams to directly communicate equipment qualification status, making it impossible to overlook PM gaps that could void validation and create audit findings through maintenance approval workflows.
Build audit confidence into every maintenance task
When maintenance documentation meets ALCOA+ principles automatically, auditors find complete evidence instead of concerning gaps. LLumin CMMS+ gives you the controlled records and validated workflows that transform GMP inspections from stress tests into verification confirmations.
Accountability for Task Completion and Review
Regulators require attribution: who performed the maintenance, who verified it, and whether qualifications were current at the time work occurred. Audit trails must show timestamps, role assignments, and any corrective and preventive action (CAPA) taken to address issues discovered during maintenance. 45% of food businesses fail their first Global Food Safety Initiative (GFSI) audit due to weak recordkeeping, with similar accountability gaps plaguing pharmaceutical operations.
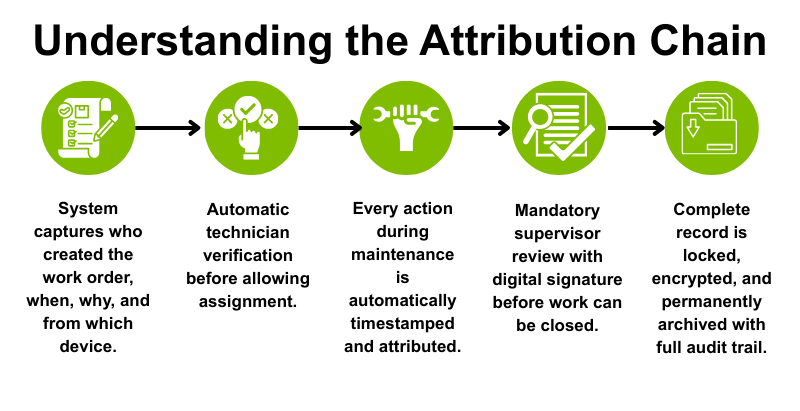
How CMMS for GMP Compliance Strengthens Performance
Procedures Embedded Directly Inside Maintenance Tasks
Technicians must follow validated, approved instructions during regulated maintenance, with no room for improvisation or undocumented steps that could affect equipment qualification. Storing procedures outside maintenance workflows increases variability and audit risk when technicians cannot immediately access current instructions. 60% of ISO 9001 nonconformities stem from documentation issues, with procedure accessibility contributing significantly to these findings.
| Procedure Management | Fragmented Systems | Integrated CMMS Platform |
|---|---|---|
| Procedure Accessibility | Binders in offices, not with the equipment | Mobile access at the point of work |
| Version Assurance | Multiple versions circulating | Single validated digital source |
| Procedure Updates | Manual notification, delays | Automatic technician retraining |
| Execution Verification | Assumed, rarely confirmed | Mandatory step-by-step completion |
Validated procedures are attached directly to every task inside sophisticated maintenance management systems, ensuring technicians follow exact approved methods. This integration supports validated maintenance procedures by making compliance the default path rather than requiring extra effort that technicians might skip under production pressure through embedded digital SOPs.
Traceability Across Maintenance, Quality, and Operations
Audit failures often stem from conflicting records between maintenance, quality, and operations teams, which undermines credibility during inspector reviews. Unified data eliminates discrepancies around what work was done, preventing inspectors from uncovering contradictory logs that trigger expanded investigations. Organizations report 48% improvement in reporting quality when using automated systems that maintain single-source data integrity.
| Cross-Functional Traceability | Siloed Department Systems | Unified Platform |
|---|---|---|
| Equipment Status Visibility | Different data in each system | Single source across departments |
| Batch-to-Maintenance Linkage | Manual reconstruction required | Automated equipment usage logs |
| Deviation Investigation | Incomplete maintenance context | Complete asset maintenance history |
| Cross-Department Traceability | Communication gaps create conflicts | Unified records eliminate discrepancies |
Teams using comprehensive platforms maintain full visibility over corrective action documentation and follow-up requirements that connect maintenance directly to product quality outcomes. This cross-functional integration ensures investigators never encounter the data conflicts that extend audits from verification exercises into compliance investigations through unified controlled maintenance records.
Master CMMS for GMP compliance through systematic maintenance
GMP audits don’t have to trigger emergency preparation. Discover how leading manufacturers maintain continuous inspection readiness through validated systems that make compliance automatic rather than aspirational.
Controls That Ensure Data Integrity and Prevent Unauthorized Changes
GMP inspectors look for ALCOA+ principles implementation: data must be attributable, legible, contemporaneous, original, and accurate throughout its lifecycle. Spreadsheets fail ALCOA+ requirements because they allow uncontrolled editing, copying, backdating, and deletion without traceable evidence of changes. Over 50% of all FDA citations relate to inadequate procedures and controls, with data integrity violations driving nearly half of all enforcement actions.
| Data Integrity Control | Spreadsheet Limitations | Validated CMMS |
|---|---|---|
| Edit Prevention | Anyone can modify historical data | Locked records after completion |
| Change Tracking | No audit trail of modifications | Complete digital audit trails |
| User Authentication | Shared logins, no attribution | Individual credentials with 21 CFR Part 11 compliance |
| Data Integrity Compliance | Fails ALCOA+ requirements | Meets all regulatory expectations |
Role-based permissions in advanced systems protect records and preserve data integrity compliance across the maintenance organization through automated controls. This technical architecture ensures regulated industry compliance by making unauthorized changes impossible rather than merely discouraged through system-enforced data protection that satisfies the most stringent regulatory standards.
Audit-Ready Documentation for Deviations and Corrective Actions
GMP requires formal documentation of equipment-related deviations and evidence that corrective actions were completed effectively before equipment returns to qualified status. Without a controlled system, deviation notes often end up isolated in emails or untracked logs that inspectors cannot verify during audits. The average food recall costs $10 million in direct expenses, with equipment-related contamination events contributing significantly when deviation management fails.
| CAPA Documentation | Manual Email Tracking | Controlled CMMS Workflows |
|---|---|---|
| Deviation Initiation | Email notifications, easily missed | Automatic work order generation |
| Root Cause Analysis | Separate documents, inconsistent | Integrated investigation templates |
| Effectiveness Verification | Often skipped or undocumented | Mandatory follow-up with evidence |
| Corrective Action Documentation | Fragmented across systems | Complete traceable records |
Corrective-action workflows in comprehensive platforms ensure CAPA documentation is linked, controlled, and fully traceable through automated processes. This systematic approach transforms equipment deviations from crisis management into controlled improvements that strengthen rather than threaten GMP compliance through structured corrective action documentation.
What Strong Digital Documentation Tells the Auditor
When auditors review maintenance records during GMP inspections, they are assessing whether the organization demonstrates actual control over equipment supporting regulated production. Strong digital documentation communicates several critical messages: maintenance activities are executed consistently, documented accurately, and reviewed through defined authority structures that prevent gaps. Equipment supporting GMP processes operates under controlled oversight with schedules, records, and responsibilities clearly defined across teams without ambiguity.
| Auditor Assessment | What Weak Records Suggest | What Strong CMMS Records Demonstrate |
|---|---|---|
| Control Over Equipment | Reactive, inconsistent approach | Systematic preventive management |
| Data Integrity | Unreliable, potentially manipulated | Complete, protected, traceable |
| Organizational Maturity | Quality as an afterthought | Quality embedded in operations |
| Inspection Readiness | Last-minute scrambling | Continuous audit-ready state |
Modern CMMS platforms bring all maintenance records into one authoritative environment that reflects real operational behavior rather than idealized procedures. This transparency supports regulated industry compliance by giving auditors immediate confidence that documentation accurately represents actual equipment control through unified GMP digital records.
Achieve Stronger GMP Reporting with LLumin CMMS+
GMP audits depend on digital maintenance records that are complete, traceable, and controlled across teams operating in regulated environments.LLumin CMMS+ delivers the controlled environment, traceability, and validation documentation that GMP operations require through CMMS for GMP compliance, designed specifically for regulated manufacturing.
Book a demo to see how LLumin CMMS+ simplifies GMP audits through unified maintenance records that demonstrate control, accountability, and continuous compliance across every regulated asset in your operation.
Frequently Asked Questions
What maintenance records do GMP auditors review during inspections?
GMP auditors review complete equipment histories, which include all maintenance events, preventive maintenance schedules with completion records, technician qualification documentation, deviation and corrective action records, and calibration certificates for measurement equipment. Over 50% of all FDA citations relate to inadequate procedures and documentation, making complete maintenance record-keeping essential. LLumin CMMS+ maintains all required documentation in one controlled system with full traceability.
How does a CMMS improve the accuracy and traceability of GMP documentation?
A CMMS improves GMP documentation through automated timestamps that ensure contemporaneous recording, digital signatures that guarantee attribution, unalterable audit trails that preserve original records, and mandatory approval workflows that enforce review requirements. Organizations report 48% improvement in reporting quality when using automated compliance systems. LLumin CMMS+ meets ALCOA+ principles automatically through system-enforced controls rather than relying on manual compliance.
What makes digital maintenance records more reliable than spreadsheets?
Digital CMMS records are more reliable because they prevent unauthorized modifications through role-based access controls, maintain complete audit trails of every change, enforce data entry through validated forms, and link records across maintenance, quality, and operations. Spreadsheets fail ALCOA+ requirements because they allow uncontrolled editing, copying, backdating, and deletion without traceable evidence. LLumin CMMS+ provides 21 CFR Part 11 compliant electronic records that satisfy regulatory requirements spreadsheets cannot meet.
What features should a CMMS include to support Good Manufacturing Practice compliance?
A GMP-compliant CMMS should include 21 CFR Part 11 electronic signature and audit trail capabilities, role-based access controls with individual user authentication, validated maintenance procedures embedded in work orders, automatic equipment history compilation, integration with calibration and training systems, and pre-formatted audit reports organized by regulatory requirement. Compliance failures have resulted in $1.1 billion in penalties over the past five years, making the proper selection of a CMMS critical for regulated manufacturers.
How does LLumin CMMS+ help teams prepare for FDA or ISO audit requirements?
LLumin CMMS+ helps teams prepare for audits by maintaining continuous inspection readiness through complete equipment histories that are instantly accessible, automated compliance dashboards showing the current PM status, pre-formatted reports that satisfy FDA and ISO requirements, integrated deviation and CAPA tracking with full traceability, and validated workflows that prove process control. The platform eliminates last-minute preparation by keeping all maintenance documentation audit-ready year-round through systematic digital records that automatically demonstrate compliance.
